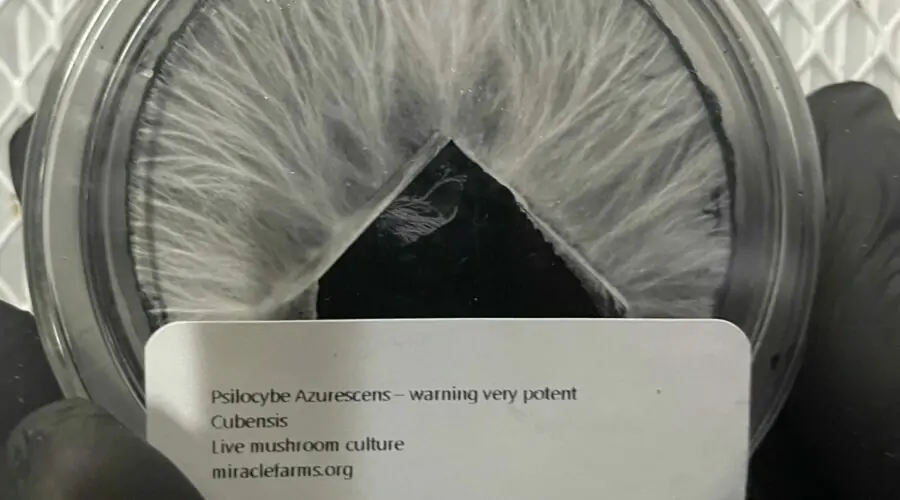
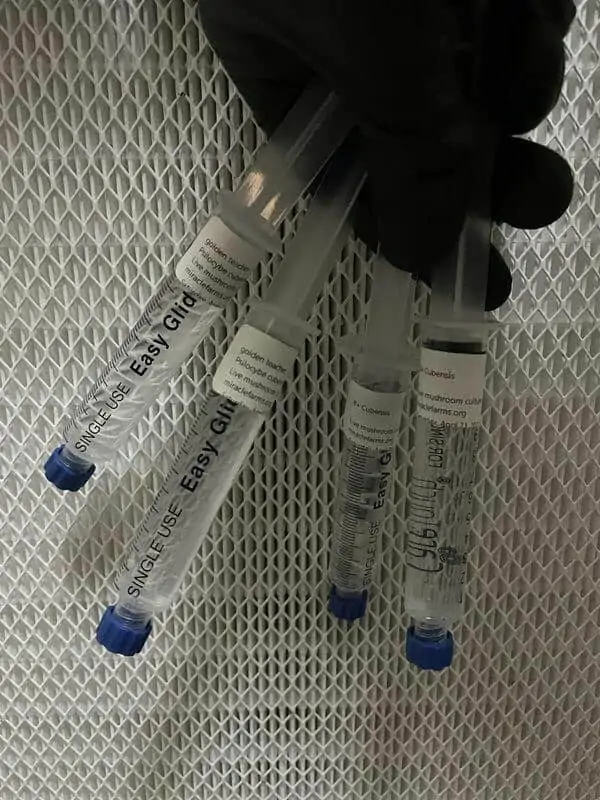
We have the hard-to-find species, the most potent, and even glow-in-the-dark cubensis, all at wholesale prices.
About my company, Miracle Farms:
Hi, my name is Brian and I am the owner of Miracle Farms. I have had a diverse career, having spent most of my life serving in the US Army. My postings have taken me to South Korea, Germany, Fort Sill OK, and Fort Bragg, NC. My area of expertise lies in the field of mycology. In 2005, I began exploring this field, and in 2010, I started my own business. All the products that I offer are made by me, by using my own tried and tested formulas developed over the years through experimentation. I do not follow the recipes of others, but instead, create my own through trial and error. I have conducted numerous experiments to perfect my products such as my mushroom pre-mix nutrient powder, agar recipes, spawn jars and bags, LC jars, etc. Once I achieve the desired outcome, I do not share the formula with anyone. While I list the most common ingredients on the label, the secret ingredients that make my products unique remain a closely guarded secret.
I include 24K Gold flakes in most of my products for two reasons. Firstly, I believe it promotes the growth of fungi, and secondly, in LC broths, the mycelium seems to use the flakes as a starting growth point. This ensures that the mycelium is distributed evenly and prevents clumping. I mention this to assure you that your sample is not contaminated. I have a collection of over 100 popular mushroom strains, and I test each one monthly, except for cordyceps strains, which are tested twice a month. I conduct a visual inspection on agar and perform DNA testing as required. All of my work is completed in the lab, under multiple laminar flow hoods. You can find further information about my company and see images of our lab on our website.











Our lab/farm in Florida is dedicated to growing over 100 gourmet strains of high-quality spores. We prioritize consistency in our products and source our psychedelic and cubensis spores from two trusted distributors in Amsterdam, Netherlands. By purchasing in bulk, we ensure a constant supply of fresh spores no older than 60 days with minimal contamination. While no company can guarantee 100% contamination-free products, our extensive industry experience allows us to come close. Our store also offers gourmet mushroom cultures, agar plates, slants, and growing equipment through our eBay and Etsy platforms. Our focus is on providing excellent customer service and receiving positive feedback. Please note that we do not sell spores or related products on eBay or Etsy.